
Generate stronger evidence by measuring health in everyday life
Run prospective, real-world research on the Evidation platform and generate evidence using our end-to-end study services.
Work with usEngage diverse, high-value populations at scale
- Access ~5M consented individuals with deep phenotyping
- Recruit rapidly for prospective, real-world studies
- Maintain long-term engagement for repeat or longitudinal measures
Understand real-world impact on daily life
- Capture patient-reported outcomes and digital behavior data
- Surface insights missed by claims or structured medical records
- Focus on how people live—not just how they're coded
Generate evidence that supports access and differentiation
- Demonstrate burden reduction, quality of life, and productivity impact
- Compare outcomes across subpopulations and real-world use
- Translate findings into decision-grade, real-world value stories
The Evidation Community: an ongoing connection to 5M people
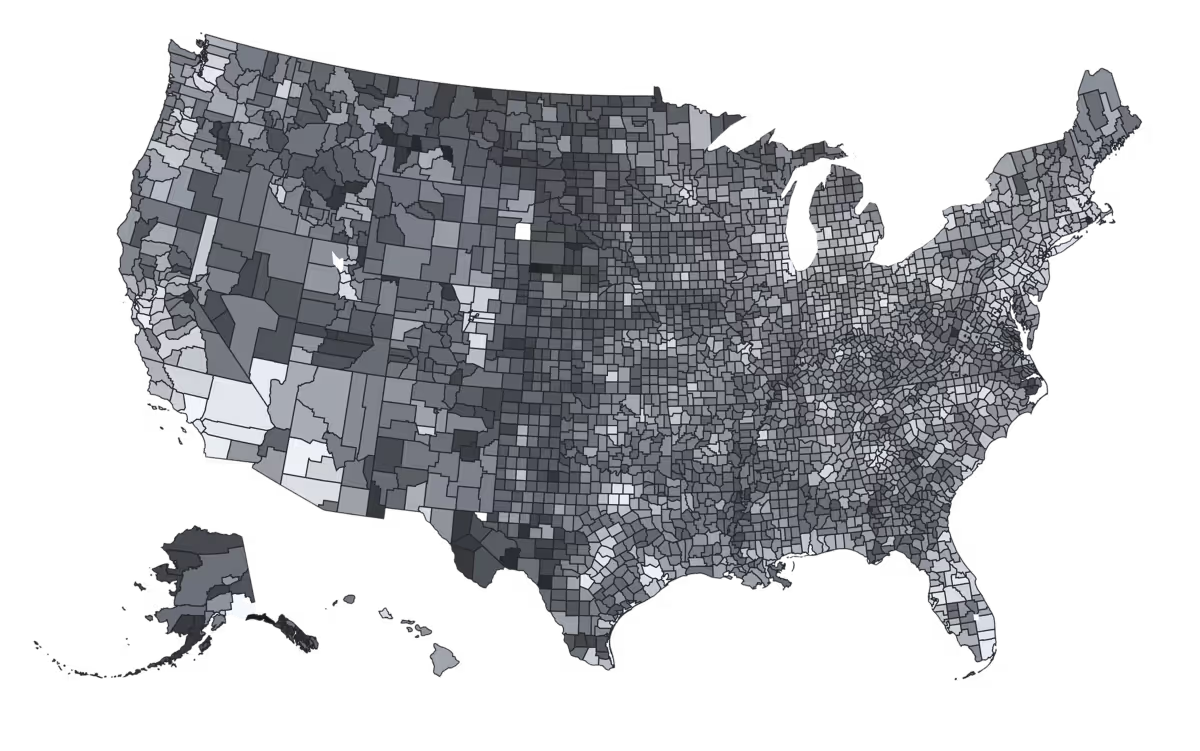
*Varies by population, protocol, and length of program; many of our recent programs have seen 90%+ engagement
**Conversion rate for “click to read” offers once offer is seen
Key features

Rapid recruitment from our highly-characterized, responsive health community— meeting specific inclusion/exclusion criteria







Enhanced participant experience and support with seamless eConsent and onboarding

Low-burden, active and passive data capture from participants, configurable to the study activity schedule



.webp)



Ongoing participant support, including intuitive, easy to understand resources
How it works

Study Design & Management
- Primary, or supporting, study services to optimize study design and the direct-to participant experience

Participant Recruitment
- Rapid and targeted recruitment from nearly 5 million members in the Evidation Community

Data Collection & Participant Support
- Low-burden, multi-modal data collection:
- ePROs and surveys
- Devices
- Health records
- At home sample collection
- Ongoing clinical support

Data Curation, Analysis & Delivery
- Curation of high volume data streams
- Structuring of data into analysis ready datasets
- Delivery of datasets and/ or analysis
- Publication support
See how we drive success for our customers:









Evidation partners include top biopharma companies , as well as consumer health companies, technology companies, non-profit organizations, and government agencies.











 work with us
work with us

















