Did you know that Evidation helps match members with relevant clinical trial opportunities?
At Evidation, we believe that contributing everyday health data to clinical trials is important to determine how well new medicines and other treatments work in real life. When solely developed and assessed in highly controlled research settings, treatments might not fit well within our often chaotic lives, especially when we’re not feeling well.
That’s why we’ve partnered with multiple organizations to provide clinical trial opportunities in areas such as diabetes, migraine, and influenza-like illnesses, so our members can provide information about their real-life experiences with their health or a new treatment.
Yet, only 44% of Evidation Members reported being aware of these opportunities in a recent survey on our app.

Clinical trials on the Evidation app
To better understand this gap in awareness, we wanted to explore how much our members know about clinical trials in general. We thought that sharing educational information about clinical trials with our members could increase knowledge and the likelihood that someone might participate in a trial with Evidation and beyond.
Therefore, in June 2023, we launched a 3-part clinical trial series in our app:
- We shared a survey to measure our members’ knowledge about clinical trials.
- Educational content was provided using videos, images and graphics, and blog posts.
- We asked members to complete a quiz to see if the educational content in Part 2 increased their knowledge about clinical trials.
Almost 7,000 of our members responded to the initial survey. Some of the results are described below.
Part 1: Knowledge about clinical trials
Only 28% of Evidation Members reported being “very familiar” or “extremely familiar” with clinical trials. So, if you feel like you know very little about clinical trials, you’re not alone!
Quick fact: What is a clinical trial?
Clinical trials look at new and different ways to prevent, detect, or treat disease. Scientists, doctors, and other specialists work with members of the public to determine if a new or improved medicine, vaccine, or other treatment works and is safe for people to use. To do this, they carefully plan the clinical trial, which is reviewed by an independent group of experts, and follow strict rules to make sure everything is done safely, ethically, and fairly. Specialized agencies, such as the Food & Drug Administration (FDA), review the final results to make sure that the new treatment is, in fact, safe and works well before the public can use it.
In addition:
61% of members reported being “likely” or “very likely” to participate in a clinical trial in the future if given the opportunity.
48% of members reported being “likely” or “very likely” to refer a friend or family member for a clinical trial
We found it interesting that, although not many of our members know much about clinical trials, they still view trials in a positive light.
We performed additional analysis to see if there are differences in the willingness to participate in a clinical trial by age and type of neighborhood. It’s often thought that older adults and people living in rural areas might find it more difficult to participate in clinical trials, and this analysis allowed us to explore the attitudes toward clinical trials for these groups.
Age
In all age groups, most people reported not being “very familiar” or “extremely familiar” with clinical trials.

Despite low levels of familiarity, most people in all age groups also believed that participation in clinical trials is important, especially Baby Boomers.

More than one-half of each age group also reported they would join a clinical trial in the future, led again by Baby Boomers, who reported the greatest likelihood.

Quick fact: Where are clinical trials run?
Evaluations and activities for clinical trials can be performed in different locations. For example, they could be completed:
- In person at a clinic.
- By a nurse in your house.
- Online or in an app on your phone or computer.
When all evaluations have to be completed in person, we call that an “in-person” trial. “Virtual” trials allow you to complete all evaluations at home, and “hybrid” trials use a combination of “in-person” and “virtual” assessments. Virtual or hybrid trials are typically considered to be more convenient because they require less travel and time away from work, family, and other responsibilities.
To determine which type of clinical trial (in-person or virtual) our members prefer, we asked:
“Imagine that Evidation notified you that you may be eligible for a clinical trial. Please rate how likely you would be to complete the eligibility screener in each of the following scenarios.”
An “eligibility screener” is a questionnaire used to determine if you meet the requirements to take part in a trial. For example:
- The trial might be only for a specific health condition or disease, like diabetes or heart disease.
- You might have to currently be non-medicated for that condition.
- You might also have to be experiencing symptoms at a certain frequency or severity.
Over two-thirds of all age groups said they were “likely” or “very likely” to participate in a virtual trial, compared with only one-quarter to one-third of members for in-person trials.
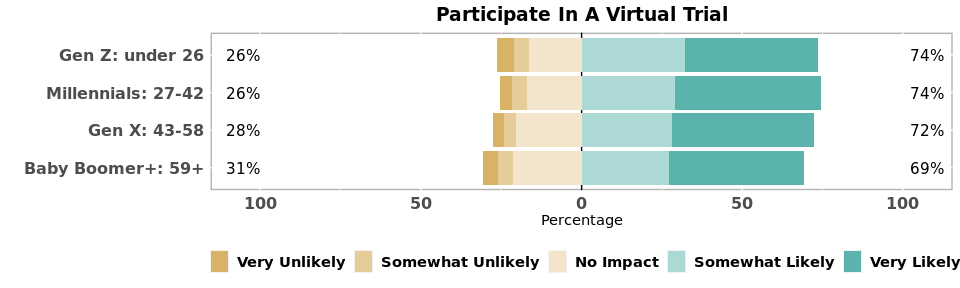

Neighborhood
Our members living in small towns or micropolitan areas were less familiar with clinical trials than those living in metropolitan areas.

Members in all neighborhood types were more likely to participate in a virtual trial than an in-person trial. Specifically, there were about 8 times more people who would “very likely” participate in a virtual trial than an in-person trial. This was true even among rural and small town members who can find it challenging to participate in clinical trials because of their distance from a research center.

Part 2: Educational content about clinical trials
Shortly after completing the initial survey, we delivered educational content about clinical trials to members. The educational content and more were presented in cards like these on our app during Part 2:

Part 3: The effect of education on knowledge about clinical trials
After reviewing the educational material in Part 2, our members’ knowledge about clinical trials improved, including:
.png)
.png)
Quick fact: Who can participate in clinical trials?
Some clinical trials focus on a specific disease or condition, while others are interested in testing the safety and effectiveness of, for example, a vaccine for everyone (healthy volunteers).
What does this information tell us?
This type of information from our members helps us understand how we can continuously improve the materials we share in our app. For example, we saw that clinical trials are generally viewed favorably by members, but a lack of knowledge about clinical trials and what’s expected might contribute to limited involvement. In addition, although the education in the app slightly improved knowledge, there is more we can do to help our members understand this topic better.
One additional learning for us was that most of our members would be more likely to participate in a virtual trial, where all study activities can be done at home. Did you know that Evidation offers many opportunities to participate in virtual trials? Look out for new recruitment offers from Evidation to see if you qualify for our upcoming studies!
Interested in joining our community to learn more about how you can participate in health research? Download the Evidation app today!











